Every cell in your body carries DNA, a molecule so small you can’t see it—yet so powerful it controls everything from eye color to enzyme production. DNA is made of two chains of nucleotides. Which type of bonds hold the chains together? That’s the key question we’re answering today.
Short answer: hydrogen bonds hold the two DNA strands together.
But if you want to actually understand how and why, keep reading. This isn’t just a memorization fact for exams. It’s the foundation of genetics, replication, and life itself.
The Structure of DNA: A Quick Refresher
Before we talk about bonds, let’s make sure we’re clear on structure.
In 1953, scientists James Watson and Francis Crick described DNA’s famous double-helix shape. Think of it like a twisted ladder.
Here’s how it’s built:
-
Two nucleotide chains form the sides.
-
Each chain has a sugar-phosphate backbone.
-
The “rungs” of the ladder are made of nitrogenous base pairs:
-
Adenine (A)
-
Thymine (T)
-
Guanine (G)
-
Cytosine (C)
-
The two strands run in opposite directions (antiparallel), forming the iconic spiral.
Now let’s answer the main question directly.
Which Type of Bonds Hold the Two DNA Chains Together?
The correct answer is:
Hydrogen bonds
If you’re choosing between:
-
Hydrogen bonds
-
Covalent bonds
-
Ionic bonds
-
Polar covalent bonds
The strands are held together by hydrogen bonds between complementary base pairs.
Here’s how it works:
-
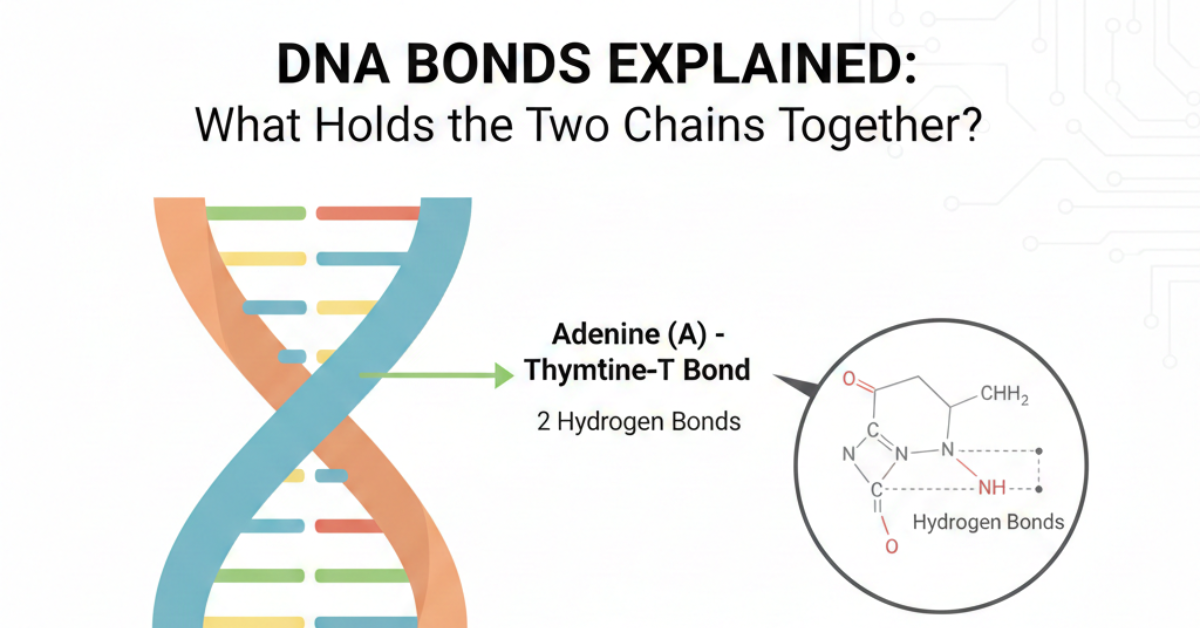
Adenine (A) pairs with Thymine (T) → 2 hydrogen bonds
-
Guanine (G) pairs with Cytosine (C) → 3 hydrogen bonds
These hydrogen bonds connect the bases across the two strands.
That’s what keeps the double helix intact.
What Are Hydrogen Bonds?
Hydrogen bonds are weak electrostatic attractions between:
-
A partially positive hydrogen atom
-
A partially negative atom (like oxygen or nitrogen)
They are much weaker than covalent bonds—but that’s intentional.
Why?
Because DNA must:
-
Stay stable
-
Yet open easily during replication and transcription
Hydrogen bonds provide the perfect balance.
What About Covalent Bonds?
Now don’t get confused.
Covalent bonds absolutely exist in DNA—but they serve a different purpose.
Covalent bonds hold together:
-
The sugar and phosphate groups in each strand
-
The bond between sugar and nitrogenous base
These are called phosphodiester bonds, and they form the backbone of each individual chain.
So remember:
-
Hydrogen bonds → between the two strands
-
Covalent bonds → within each strand
That distinction is critical.
Why Not Ionic Bonds or Polar Covalent Bonds?
Let’s break it down clearly.
Ionic Bonds
Ionic bonds involve full electron transfer and charged ions. DNA base pairing doesn’t work this way. So this option is incorrect.
Polar Covalent Bonds
Polar covalent bonds share electrons unequally, but these form within molecules—not between the two DNA strands holding them together.
So again, the correct answer remains:
Hydrogen bonds hold the two DNA chains together.
Why Hydrogen Bonds Are Biologically Brilliant
Here’s the genius of the design:
-
They are weak individually
-
But strong collectively
-
They allow easy strand separation
-
They enable accurate base pairing
G-C pairs form three hydrogen bonds, making them stronger than A-T pairs (two bonds). That’s why DNA regions rich in G-C are harder to separate.
This matters in:
-
DNA replication
-
PCR testing
-
Gene expression
-
Thermal stability studies
How DNA Unzips During Replication
When cells divide, DNA must copy itself.
Enzymes break the hydrogen bonds between base pairs. Because hydrogen bonds are relatively weak, this can happen without destroying the entire molecule.
If those were covalent bonds instead?
Replication would be nearly impossible without damaging the genetic code.
That’s the beauty of molecular biology—precision engineering at the atomic level.
Summary Table: DNA Bond Types
| Bond Type | Location | Function |
| Hydrogen bonds | Between base pairs | Hold two strands together |
| Covalent bonds | Sugar-phosphate backbone | Form each individual strand |
| Phosphodiester bonds | Within backbone | Connect nucleotides |
| Ionic bonds | Not used in strand pairing | Not responsible for DNA structure |
FAQs
1. DNA is made of two chains of nucleotides. Which type of bonds hold the chains together?
Hydrogen bonds between complementary nitrogenous bases (A-T and G-C).
2. Are covalent bonds involved in DNA?
Yes. Covalent bonds form the sugar-phosphate backbone of each individual strand.
3. Why are hydrogen bonds important in DNA?
They allow DNA strands to separate easily during replication while still maintaining overall stability.
4. Which base pair has more hydrogen bonds?
Guanine–Cytosine pairs have three hydrogen bonds, while Adenine–Thymine pairs have two.
5. What happens if hydrogen bonds break?
If temporarily broken during replication, it’s normal. If disrupted permanently (e.g., extreme heat or chemicals), DNA can denature.
Conclusion
Let’s make it crystal clear:
DNA is made of two chains of nucleotides. The type of bonds that hold the chains together are hydrogen bonds.
Covalent bonds build each strand. Hydrogen bonds connect the two strands. That separation of roles is what makes DNA stable yet flexible enough to support life.
If you’re studying biology, prepping for exams, or just curious about how genetics works, understanding this concept is non-negotiable.
Want to go deeper? Next, explore:
-
DNA replication mechanisms
-
RNA structure vs DNA
-
Mutations and genetic variation